Millitransflow Fluidic System
In vitro barrier models are helpful tools in preclinical research to evaluate the uptake and transport properties of drug candidates. One often-used approach to study transepithelial transport utilizes cell layers cultured on the porous membrane of transwell inserts. Test compounds are introduced into the apical compartment and transported through the barrier into the basolateral compartment of the transwell device. As we detail below, the Millitransflow Fluidic System automates several aspects of transport experiments.

The Millitransflow Fluidic System efficiently manages culture media in a transwell device's apical and basolateral chambers. It can also introduce test compounds into the apical compartment at a pre-determined phase of the experiment. The system removes and stores media for subsequent analysis in a refrigerated fraction collector. Test compounds and fresh and collected media are transported through distinct tubing systems, minimizing the risk of cross-contamination. Between successive measurements, the tubing network undergoes automated cleaning and disinfection procedures overnight, ensuring the reliability of the research processes.
Outside the cell culture incubator, a dosing unit incorporates two syringe pumps and a tube multiplexer to measure and inject cooled media into the incubator unit precisely. The freshly injected medium is further conditioned in a CO2-permeable heat exchanger within the incubator for 30 minutes before being introduced into the culture. Remarkably, the transition from removing the existing apical or basolateral medium to injecting fresh medium occurs in less than 10 seconds.
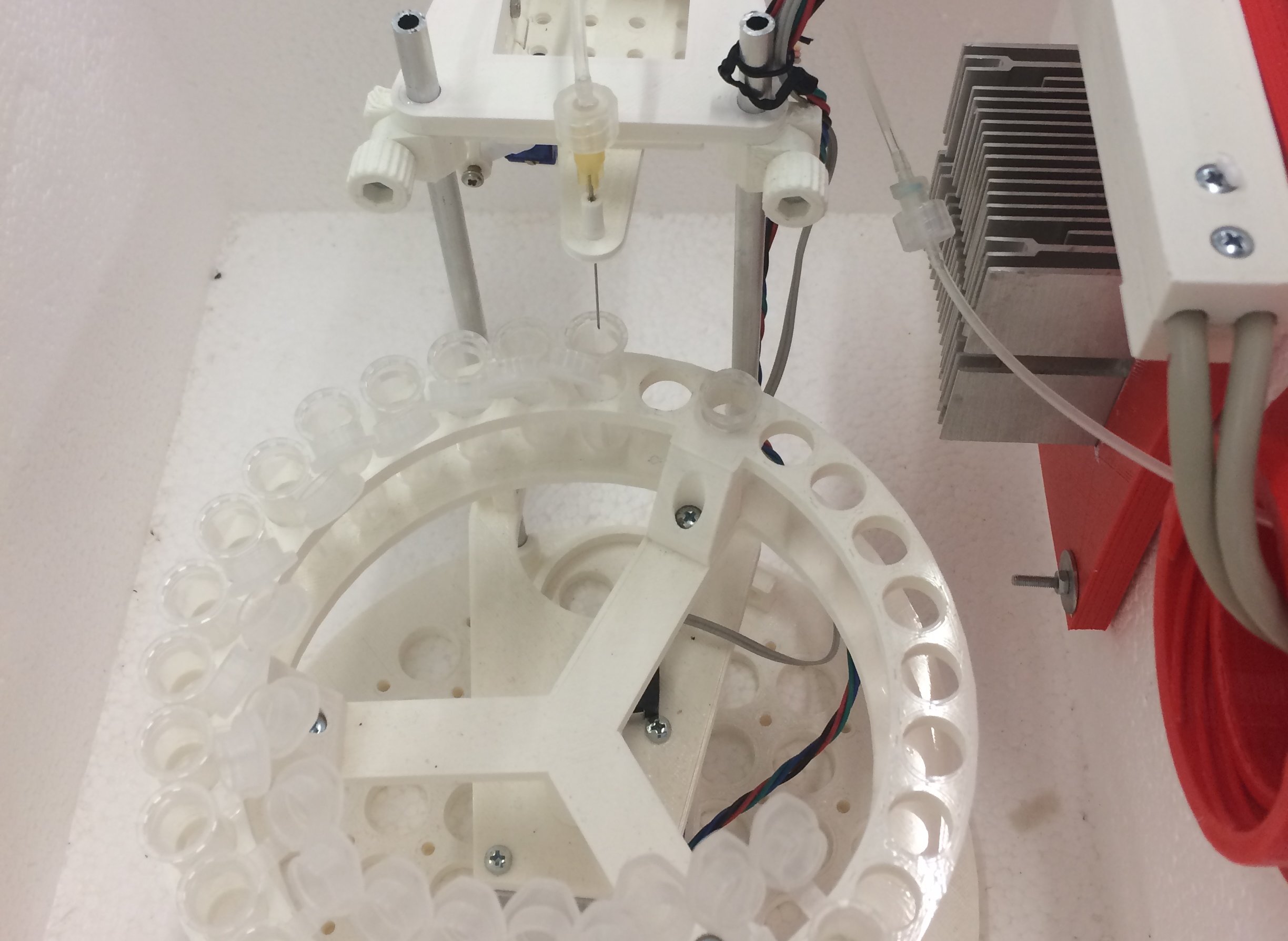
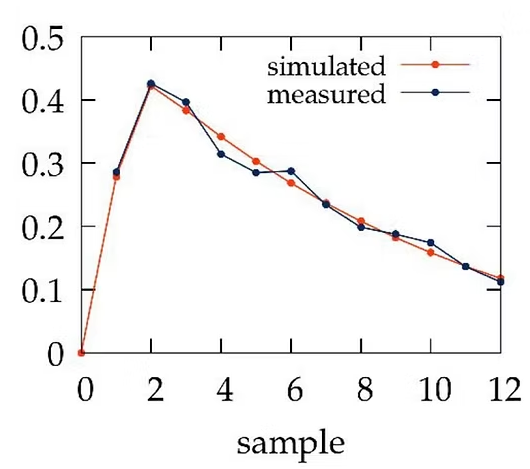
Automated sample collection with the Millitransflow Fluidic System can determine the time-resolved transport kinetics of various test compounds. A detailed mathematical model of the sample collection process can estimate and compensate for adsorption artifacts, thus providing a more accurate estimate of diffusive transport through the transwell insert. Time-resolved concentration data of the test compound allows for determining parameters of the cellular barrier, such as the diffusive permeability of the test compound, its affinity to the cell membrane, its diffusivity through the cellular barrier, and the rate of its metabolic elimination.
References:
This product incorporates the intellectual property of researchers at Eötvös Loránd University.
